The greenhouse effect, calculated again
I did not want to talk about the greenhouse effect without having checked the math and physics. Therefore I calculated it myself.
If you want all links to work, read the the PDF-version.
The greenhouse effect describes the effect of the atmosphere on Earth’s surface temperature. The simplest example contrasts the surface temperature of a planet without atmosphere to the surface temperature with a single insulating layer above the surface.
The incoming radiation from the Sun provides the earth with a constant source of energy. If it would not get rid of that energy somehow, it would get hotter everyday, eventually melt and vaporize. It’s evident that this does not happen (otherwise we would not be here to think about it).
As shown by \citet{Stefan1879} and \citet{Boltzmann1884}, the total energy emission from a perfect black body (a body which absorbs all incoming radiation) per unit area is given by
\begin{equation} E = \sigma T^4 \end{equation}with the Stefan–Boltzmann constant
\begin{equation} \sigma = \frac{2 \pi^5 k^4}{15 c^2 h^3} \approx 5.67 \cdot 10^{-8} W m^{-2} K^{-4} \end{equation}From satellite measurements in the Earth’s orbit we know that the incident solar radiation delivers an average energy flux \(j\) between 1361 \(Wm^{-2}\) during the solar minimum and to 1363 \(Wm^{-2}\) during the solar maximum \citep{KoppSolarConstant2011}.
This radiation hits the cross section of the Earth, the area of a circle with the radius of the Earth: \(\pi R^2\). This is also the energy radiated by the Earth system, as evidenced by the Earth neither melting nor freezing. But this outgoing radiation is perpendicular to the surface, not to the cross section of the Earth. The total surface of a sphere is \(4 \times \pi R^2\), or \(4 \times\) its cross section. So the energy radiated per area is just 25% of the incoming energy: \(σT_{out}^4 = 0.25 \times σT_{in}^4\)
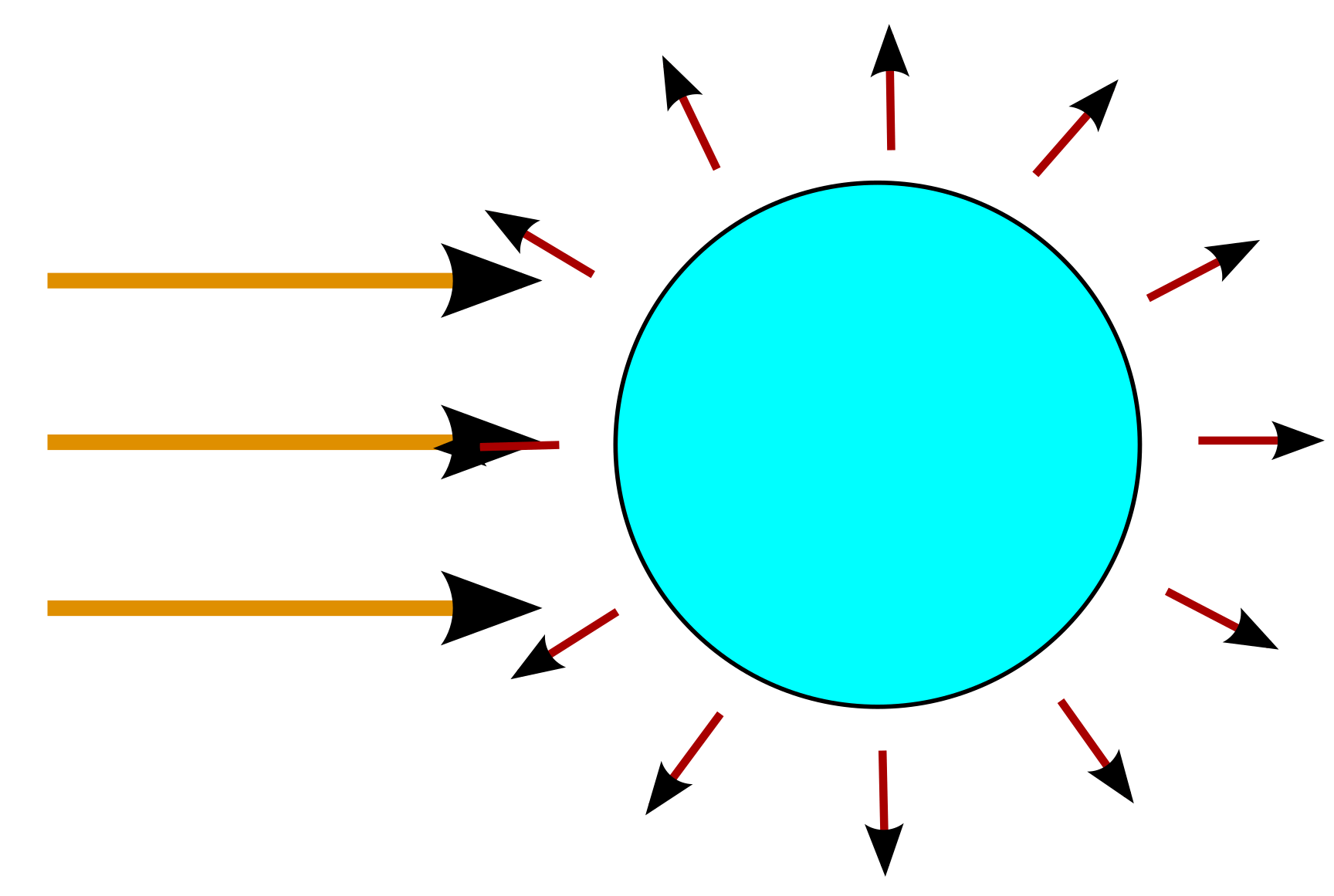
The incident radiation delivers an Energy flux of \(E_i = 1362 Wm^{2}\), so the outgoing radiation of a perfect black body would be \(E_o = 340.5 Wm^{-2}\), which is consistent with a temperature of
\begin{equation} T = \left(\frac{E}{σ}\right)^{\frac{1}{4}} = \left(\frac{340.5 \cdot 15 c^2 h^3}{2 \pi^5 k^4}\right)^{\frac{1}{4}} K \approx \left(\frac{340.5}{5.67 \cdot 10^{-8}}\right)^{\frac{1}{4}} K = 278.623 K \end{equation}This gives an average surface temperature of
\begin{equation} (278.62 - 273.15) ^\circ C = 5.47 ^\circ C \end{equation}for a perfectly black Earth without atmosphere.
Due to the simplifications used, this value is \(8 K\) lower than the measured mean sea and land surface temperature of \(14 ^\circ C\) for the base period 1961-90 \citep{Jones1999,Rayner2006}.
Historically the next step after the black body estimation was to take the albedo into account: The amount of incoming radiation reflected directly back into space.
If we take into account that the Earth surface and clouds reflect roughly 30% of the visible light back into space, the Earth only receives roughly 70% of the energy which it needs to radiate back. For details, see \citet{Muller2012} and \citet{Muller2013}.1 The equilibrium temperature changes to 255 Kelvin, which is just about -18 °C. Note that changing the albedo by 1 percent point (to 29% or 31%) would change the temperature by roughly 1 K.
(let* ((albedo 0.3)
(sol 1362)
(incoming-watt (* (- 1 albedo) (/ sol 4)))
(c 3e8)
(h 6.62607e-34)
(k 1.38065e-23)
(pi 3.14159))
(expt
(/ (* incoming-watt 15 c c (expt h 3))
(* 2 (expt pi 5) (expt k 4)))
0.25))
254.61953320379396\begin{equation} T = \left(\frac{E}{σ}\right)^{\frac{1}{4}} = \left(\frac{\color{red}0.7\color{black} \cdot 340.5 \cdot 15 c^2 h^3}{2 \pi^5 k^4}\right)^{\frac{1}{4}} K \approx \left(\frac{238.0}{5.67 \cdot 10^{-8}}\right)^{\frac{1}{4}} K = 254.6 K \end{equation}
There are small additional factors in play:
- effective temperature (radiation from a star) to air temperature (measured on Earth)
- emissivity: Common values range from 0.90 to 0.98, with forests and urban areas staying close around 0.95, grassland peaking at 0.95 but with a noticeable tail towards 0.90 and barren soil and sparsely vegetated areas forming a broad distribution between 0.92 and 0.96 \citep{Jin2006}. Snow 0.99 (Wan2002).
But with these we’re still roughly 30 Kelvin away from actual temperatures. These are reached through absorption and radial re-radiation of outgoing energy, which effectively provides the Earth with insulation, most effective in the infrared.
This is what is typically called the greenhouse effect: Infra-red emissions by the Earth are absorbed by greenhouse gases in the atmosphere, so the Earth needs to be warmer to get rid of the same amount of received energy.
Greenhouse gases have a net effect on the temperature, because the outgoing radiation mostly consists of thermal infrared light (TIR), while the incoming radiation mostly consists of near infrared (NIR) visible (VIS) and ultraviolet (UV) light. Let’s take the oldest account of this absorption: \citet[“On the Influence of Carbonic Acid in the Air upon the Temperature of the Ground”]{Arrhenius1896} describes different absorption of moonlight depending on the wavelength of the light. Half of the energy of this light absorbed in the atmosphere is radiated outwards, the other half inwards.
For the actual calculation, we use more recent results: \citet[“lThe natural greenhouse effect of atmospheric oxygen (O2)and nitrogen (N2)”]{Hoepfner2012}. They take into account the structure of the atmosphere by building on the well-established Karlsruhe Optimized and Precise Radiative transfer Algorithm (KOPRA).
The publication by \citet[]{Hoepfner2012} showed that Outgoing Longrange Radiation without gas would be 365.7 W/m\(^2\), while with greenhouse gases it is 242.7 W/m\(^2\) (calculated with KOPRA that’s used to analyze satellite data and \citet[“has successfully been compared to various independent radiative transfer models”]{Hoepfner2012}). That’s a 33.6 % decrease in emission, so we need 1.5 times higher emissions to reach equilibrium. Let’s factor this into the equations, and also use an emissivity of 0.95 for the earth’s surface in infrared.
(let* ((albedo 0.3) (emiss 0.95) (sol 1362) (incoming-watt (* (- 1 albedo) (/ sol 4))) (c 3e8) (h 6.62607e-34) (k 1.38065e-23) (pi 3.14159)) (expt (/ (* (/ 365.7 242.7) incoming-watt (/ 1 emiss) 15 c c (expt h 3)) (* 2 (expt pi 5) (expt k 4))) 0.25)) ;; for the numerator ;; (* (/ 365.7 242.7) (/ 1 emiss) incoming-watt))
285.7423501045961\begin{equation} T = \left(\frac{E}{σ}\right)^{\frac{1}{4}} = \left(\frac{\frac{365.7}{242.7} \cdot \color{red}0.7\frac{1}{0.95}\color{black} \cdot 340.5 \cdot 15 c^2 h^3}{2 \pi^5 k^4}\right)^{\frac{1}{4}} K \approx \left(\frac{377.49}{5.67 \cdot 10^{-8}}\right)^{\frac{1}{4}} K = 285.74 K \end{equation}
We get 285.74 K as equilibrium temperature. That’s around 12.6°C, so now we’re just 1.4 Kelvin away from the actual \(14 ^\circ C\) for the base period 1961-90 \citep{Jones1999,Rayner2006}. There are still effects missing in the calculations, but the intention of this guide is not to create a new climate model, but to show the fundamental physical effects. Remember also that changing the surface albedo by 1 percent point (to 29% or 31%) would change the temperature by roughly 1 K, so getting within less than 2 °C of the measured temperature is already pretty good. Going further would require a much stricter treatment of surface albedo that goes into too much detail for an article.
Therefore we’ll round this up with an important test that is only weakly affected by the surface albedo:
What happens if we increase the absorption by CO\(_2\)? Do we see global warming?
For this test the result is already close enough to the measured temperature that we can take the difference between values with different parameters to get the effect of these parameters and remove biases which are present in both values.
To calculate global warming due to doubled CO₂, we cannot just double the absorption, because the absorption bands get saturated. The \citet[IPCC working group 1 (physical science basis)]{IPCCRadiativeForcingMyhre2013} gives the increase in radiative forcing due to increased CO\(_2\) levels from the 1950 concentrations of about 310 ppm to the 2010 concentrations of 390 ppm as about 1.2 W/m\(^2\).2
So let us go at this backwards: \citet{Hoepfner2012} showed the state for 2012, what do our calculations predict for 1950 when we reduce the absorption due to CO\(_2\) by the 1.2 W/m\(^2\) radiative forcing given in the IPCC?3
The unstable emissions would then not be 242.7 W/m\(^2\) as calculated by \citet{Hoepfner2012}, but 243.9 W/m\(^2\)
(let* ((albedo 0.3) (sol 1362) (emiss 0.95) (incoming-watt (* (- 1 albedo) (/ sol 4))) (c 3e8) (h 6.62607e-34) (k 1.38065e-23) (pi 3.14159)) (expt (/ (* (/ 365.7 (+ 242.7 1.2)) incoming-watt (/ 1 emiss) 15 c c (expt h 3)) (* 2 (expt pi 5) (expt k 4))) 0.25)) ;; for the numerator ;; (* (/ 365.7 (+ 242.7 1.2)) incoming-watt (/ 1 emiss)))
285.3902331695058\begin{equation} T = \left(\frac{E}{σ}\right)^{\frac{1}{4}} = \left(\frac{\frac{365.7}{243.2} \cdot \color{red}0.7\frac{1}{0.95}\color{black} \cdot 340.5 \cdot 15 c^2 h^3}{2 \pi^5 k^4}\right)^{\frac{1}{4}} K \approx \left(\frac{375.64}{5.67 \cdot 10^{-8}}\right)^{\frac{1}{4}} K = 285.39 K \end{equation}
We get 285.39 Kelvin for 1950, about 0.35°C less than for 2010.
This gives an estimate of a 0.35°C increase in temperature from 1950 to 2010 due to increased CO\(_2\) levels alone. If we also remove the added absorption from methane, N\(_{2}O\) and other greenhouse gases emitted by humans (additional forcing of 0.75 W/m\(^2\)), we get 285.17 Kelvin.
So this calculation from basics yields an increase of the equilibrium temperature by 0.57 °C.
\begin{equation} T_{2010} - T_{1950} = 285.74 K - 285.17 K = 0.57 K \end{equation}This is a bit lower than the increase of 0.65 K to 0.75 K seen in the global temperature records by the Berkeley Earth project,4 and close to the 0.6 to 0.8 K increase shown in the Global (NH+SH)/2 temperature given by HadCRUT4 by the Met Office Hadley Centre by the National Meteorological Service of the United Kingdom.5 But for a calculation from basic principles, that’s pretty good.
So we can conclude that actual measurements match this physical explanation of global warming due to the greenhouse effect — or more exactly: due to increased absorption of infrared radiation by greenhouse gases, with the biggest effect due to CO\(_2\).
The source of climate-active human carbon emissions which influences the CO\(_2\) content of the atmosphere is mostly burning of fossil fuel which is taken from the crust of the Earth and introduced into the carbon cycle. This is what changes the CO\(_2\) concentration.
And with this, we are done.
Please reduce your carbon emissions and become active to get politicians to action on a national and global scale. We’re cutting the branch we live on.
If you want more details, have a look at the IPCC reports. Best start with the executive summary and then go into the details you’re most interested in:
IPCC Climate Change 2013: The Physical Science Basis: https://www.ipcc.ch/report/ar5/wg1/
An explanation how humans increase the CO₂-concentration of the atmosphere is available in my presentation The carbon cycle: https://www.draketo.de/licht/physik/kohlenstoffkreislauf-carbon-cycle
And if you want my best estimate of our current situation, have a look at the article Two visions of our future: https://www.draketo.de/english/politics/roll-a-die
Fußnoten:
You can check the albedo for several different spectral regions at http://www.globalbedo.org/
Robust data about changing CO\(_2\) levels is available from the Global Monitoring Division (GMD) of the Earth System Research Laboratory (ESRL) at the National Oceanic & Atmospheric Administration (NOAA).
We’re only going to 1950 and not back to 1850, because the temperature data at 1850 would mix in the effect of the declining little ice age.
Berkeley Earth provides a reevaluation of all the surface measurements without complex models.
HadCRUT4 combines sea surface temperature data from the Hadley Centre of the UK Met Office and the land surface air temperature records compiled by the Climatic Research Unit (CRU) of the University of East Anglia.
